It can smell sickly (as in, um, vomit), or sweet, even added to perfumes. It can be found in certain foods, produced by our bodies or taken as a supplement.
A quick Google search reveals the main claims of benefit for Butyric Acid (BTA), also known as Butanoic Acid, relate to gut health and improving gut conditions. This includes conditions such as Irritable Bowel Syndrome, Crohn’s Disease, and Colorectal Cancer.
Is there evidence to back these claims and, if so, how strong is it? I looked in to six of the common claims for BTA.
What Is Butyric Acid?
BTA is a saturated, short-chain fatty acid (SCFA) with a 4-carbon backbone. (1) The salts and esters of BTA are known as butyrate’s (BT) or butanoate’s, with the terms often used interchangeably.
An ester is a compound produced by the reaction between an acid (in this case, BTA) and an alcohol. Methyl butyrate is an example of a BTA ester. It is found in alcoholic beverages and in many fruits, such as apple juice, apricot, blackberry, and nectarine. It’s also present in cheese, butter, milk, and other dairy products, as well as white wine, coffee and black tea. (19, 6) As the glycerol ester, BTA is present in animal fats, rancid butter, parmesan cheese and plant oils. (17)
Interestingly, BTA even makes an appearance in vomit - it has an unpleasant odor and acrid taste, with a sweetish aftertaste (similar to ether). (17) Yet, low-molecular-weight esters of BTA, such as methyl butyrate, have mostly pleasant aromas or tastes. For this reason, they may be used as a food flavoring ingredient, and a perfume additive, and are F.D.A. approved as such. (19)
BTA is also a by-product of the carbohydrates that we consume, such as dietary fiber. During the fermentation of the carbohydrates by our gut bacteria in the large intestine, SCFA metabolites are produced and serve as a key energy source. (17) BT is one of the main SCFA’s made during this process. (2)
It can also be taken as a supplement. BTA itself, as an acid, can be corrosive, and in supplements it will be found in a salt form (eg. sodium butyrate).
The current research
I ran a search on the associated keywords ‘Butyric Acid’, ‘Butanoic Acid’, ‘Butyrate’ and ‘Butanoate’, for available papers in the English language. For papers where one of these keywords is included in the title (and therefore a main focus of the paper), there are less than 4000-indexed papers. Of these, about 1600 of them relate to humans, specifically, with no meta-analyses and 3 systematic reviews available.
Let’s compare that to a better known gastrointestinal supplement, such as a ‘probiotic’ (and including keyword ‘prebiotic’ in the search). For papers where it is a main focus, there are about 5500-indexed papers, with over 2000 of them related to humans.
Most impressive is the amount of quality evidence available – of those 2000 papers specific to humans, 37 are meta-analyses and 69 are systematic reviews. One could be quite confident in drawing a firm conclusion from such available evidence.
Now, let’s return to BTA. In the following categories, specifically, how much evidence is available? Powerful meta-analyses and systematic reviews are focused on, as usual and, where available.
Does Butyric Acid Assist Weight Loss?

In this category, I found one 2017 systematic review. It looked at weight-loss interventions and gut microbiota changes in obese and overweight patients, but did not offer any conclusive evidence relating to BTA’s role. (4)
There are some small animal studies available. A study in mice, that were fed a variety of SCFA, found that butyrate “protected against diet-induced obesity”. The method by which this was achieved was via “Stimulation of gut hormones and food intake inhibition”. (2) Another study in mice fed a high-fat diet, found that the development of obesity was prevented through supplementation with sodium butyrate. They concluded that butyrate achieved this partly through “promotion of energy expenditure”. (3)
When widening the search to SCFA, neither meta-analysis nor systematic reviews are available. Three available reviews did not shed much evidence to the topic at hand.
Bottom Line: At this time, virtually no evidence of a role for BTA in human weight loss. Animal studies offer limited evidence, though better quality research is needed to be conclusive.
Is It A Potential Colorectal Cancer Treatment?
BT is formed in the human colon as a product of fiber fermentation, and this is suggested as a factor explaining why high fiber diets are protective in preventing colorectal cancer (CRC). (17)
Although there are a couple of meta-analyses and systematic reviews available, CRC was not the main topic being reviewed. A 2011 systematic review noted that, “a growing number of studies have stressed the role of butyrate in the prevention and inhibition of CRC”. (5) A further systematic review further comments that butyrate shows “promising effects in.. colorectal cancer”. (6)
However, individual clinical trials have shown mixed results regarding the anti-tumor effects of butyrate. (7) In humans, the relationship between luminal butyrate exposure and CRC has been examined only indirectly, in case-control studies, by measuring fecal butyrate concentrations. Results of these investigations have been mutually contradictory.
This, though, may not accurately reflect effective butyrate exposure during cancer formation. The direct effect of butyrate on tumor formation has been assessed in a number of in vivo animal models, which have also yielded conflicting results. (18)
One promising 2015 study, analyzing human CRC cell lines treated with sodium butyrate at concentrations ranging from 0.5-5 mM, found that it inhibited the growth of the studied cancer cells, stimulated autophagy and caused cell death. (7) This may reveal one way BTA may potentially exert anti-cancer activity.
Mouse studies reveal another way that BTA may act; showing a 75% reduction in the risk of CRC in animals fed a fiber-rich diet. The mechanism involved colonic bacteria, which produce butyric acid, acting as intermediaries, compared to bacteria-free mice. They concluded that this type of bacteria was specifically required for a fiber-rich diet to exert is anti-cancer effects. (8)
Bottom Line: There have been conflicting results, to date, of a role for BTA in cancer treatment in both human and animal studies.
Does It Provide Relief to Irritable Bowel Syndrome (IBS)?
BTA is thought to play quite a few important roles in the gastrointestinal tract (GIT). IBS is the most commonly diagnosed functional GIT condition, with about 12% of adults experiencing IBS symptoms (with other studies estimate this percentage to be considerably higher, and increasing). Since the cause of IBS is unclear, treatment focuses on symptom relief such as bloating, abdominal pain, diarrhea and constipation. (1)
Are there any meta-analyses or systematic reviews available suggesting a role for BTA on IBS management? Not that I could find. There are a few reviews, though, in the approximately forty papers available in this category.
One study randomized 66 IBS patients to receive either supplemental micro-encapsulated sodium butyrate (MSB) (34 patients) or placebo (32 patients), in addition to their standard pharmacological IBS therapy, for a duration of 3 months. After 4 weeks there was a significant decrease of pain during defecation in the MSB group which extended to improvement of urgency and bowel habit at 12 weeks. Reduction of abdominal pain, flatulence and disordered defecation was not statistically significant. (9)
In another study, the microbiomes of 113 IBS patients, and 66 control subjects, were analyzed over a couple of months. Their findings showed that the IBS patients had a significant reduction of butyrate-producing bacteria that are known to improve intestinal barrier function. (10) There are a number of other human clinical trials, albeit, small in size and short in duration, that have also demonstrated positive effects.
Bottom Line: Evidence does exist of a role for BTA in the management of human IBS symptoms. However, the studies have been relatively small-scale and better quality research is pending.
Is It A Treatment For Crohn’s Disease (an Inflammatory Bowel Disease)? Does it Generate Anti-inflammatory Effects, in general?
A couple of systematic reviews exist here. They didn’t offer specific evidence of a role for BTA as a treatment for Crohn’s Disease but a 2015 systematic review observed, “a decrease in butanoate... (and) lower levels of butyrate” in Crohn’s disease patients, among other functional changes. However, they concluded that, “Larger, prospective, and longitudinal studies are required ... if causality is to be determined.” (11)
A 2014 SR analyzed 23 randomized, controlled trials assessing the role of fiber in the treatment and maintenance of Inflammatory Bowel Disease. It included 12 trials specific to Crohn’s disease, and in none of them was fiber supplementation found to benefit disease outcomes. (12) BTA is one of the main SCFA produced during fiber fermentation in the gut.
Likewise, neither meta-analyses nor systematic reviews are available suggesting BTA as having anti-inflammatory effects in humans, though small-scale animal studies do exist. (13)
Bottom Line: No credible evidence of a role for BTA in the treatment of human Crohn’s Disease, or anti-inflammatory effects in humans, in general. Animal studies suggest there may be a role.
Does It Combat Insulin Resistance?
Again, there are neither definitive meta-analyses nor systematic reviews for the role of BTA in managing insulin resistance.
In one animal study, a single juvenile diabetic rat had sodium butyrate administered intravenously (500mg/kg/day) for 21 days. The researchers found “improved plasma insulin levels” in this rat compared to control. (14)
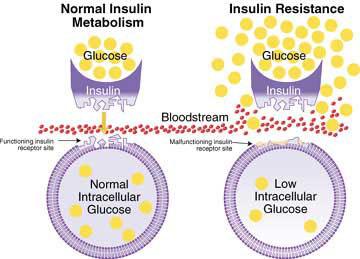
Another study in mice fed a high-fat diet found that the development of insulin resistance was prevented through supplementation with sodium butyrate. They concluded that butyrate achieved this partly through “promotion of energy expenditure”. (3)
Bottom Line: Poor evidence of a role for BTA in combating insulin resistance, overall.
Are There Risks or Side-effects To Taking Butyric Acid?
It seems to be safe in supplemental form, and even has been tested in I.V. form.
A recent clinical trial administered 300mg daily of micro-encapsulated sodium butyrate in 30 patients with diverticulosis, for a duration of 12 months. No side-effects were reported during treatment. (16)
A 1977 study infused rats with monobutyrin continuously for 7 days at 27g/kg/day. All experimental animals were fund to survive “in good health” with no “detectable physiological and behavioral abnormalities”. They concluded that monobutyrin “produces no obvious toxic affects during short infusion periods”. (15)
However, inhalation of BTA liquid, as an acid, does have recorded side-effects (but is beyond the scope of this article). (17)
Conclusion
Yes, the body of knowledge about BTA is growing. The mechanisms by which it affects the relationship between the microbiome and the host are becoming increasingly understood. At this time, however, conclusive evidence, especially in humans, is limited. Until we know more, you may wish to simply adopt, or continue eating, a high-fiber diet and sprinkling that parmesan cheese on your pasta!

Leave a Reply