Although many Americans only know bacopa as a plant found in aquariums, Bacopa monnieri has been used as a medicinal herb in Ayurvedic Medicine for many hundreds of years. It’s been used for a variety of health conditions including memory and brain-related disorders, anxiety, asthma, and gastrointestinal ailments.

People also take bacopa to treat backache, hoarseness, mental illness, epilepsy, joint pain, and sexual performance problems in both men and women. In addition, it is sometimes used as a “water pill.”
But what does the scientific evidence show about bacopa? Can it really improve cognitive function and cure depression and schizophrenia? Let’s examine what we know so far.
Table of Contents
What is Bacopa?
Bacopa monnieri is a perennial, creeping herb native to the wetlands of southern and Eastern India, Australia, Europe, Africa, Asia, and North and South America. It has succulent leaves and small white or purple flowers.
Other names for bacopa include thyme-leafed gratiola, water hyssop, Indian Pennywort, Jalanimba and herb of grace. In traditional Ayurveda, it is known as "Brahmi," after Brahmā, the creator God of Hinduism. Be careful not to confuse brahmi (Bacopa monnieri) with gotu kola and other natural medicines that are also sometimes called brahmi.
Bacopa monnieri has been used in traditional Ayurvedic medicine as well as in Traditional Chinese medicine. The first written evidence of its use was in the 6th century A.D in texts such as the Charaka Samhita, Atharva-Veda, and Susrut Samhita as a “Medhya rasayana”– a class of herb taken to sharpen intellect and attenuate mental deficits. More recently, it is being studied as a nootropic herb, which means that it can help repair damaged neurons and improve brain function.
Bacopa is available in several forms, including powder, capsules, syrup, and tea. The standard dose is 300mg. Traditionally, it is consumed with some form of animal fat, so it’s probably best to have it with meals.
Bacopa contains several biologically active compounds. These include alkaloids (brahmine, nicotine, and herpestine) saponins (monnierin, hersaponin), sterols (b-sitosterol, stigma-sterol), d-mannitol, and betulinic acid.[1] The main components of bacopa are triterpene saponins, which have been named bacosides and bacopasaponins.
There are two types of saponins- jujubogenin and pseudojujubogenin. The main saponins in bacopa believed responsible for pharmacologic effects on the nervous system are called bacoside A and bacoside B (which are derived from jujubogenin and pseudojujubogenin). A standardized extract of bacopa contains a minimum of 55%± 5% bacosides.
A Brief Primer on Neurotransmitters
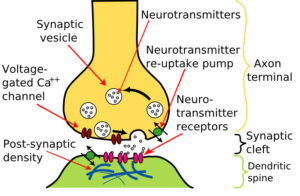 Neurotransmitters are chemicals made by nerve cells used to communicate with other cells. It is a type of chemical messenger which transmits signals across a chemical synapse, such as a neuromuscular junction, from one neuron (nerve cell) to another "target" neuron, muscle cell, or gland cell.
Neurotransmitters are chemicals made by nerve cells used to communicate with other cells. It is a type of chemical messenger which transmits signals across a chemical synapse, such as a neuromuscular junction, from one neuron (nerve cell) to another "target" neuron, muscle cell, or gland cell.
Neurotransmitters are released from synaptic vesicles in synapses into the synaptic cleft, where they are received by neurotransmitter receptors on the target cells (See diagram). The release of a neurotransmitter from one neuron can either activate or inhibit a second neuron.
There are six main neurotransmitters in the brain. They are outlined in the chart below:

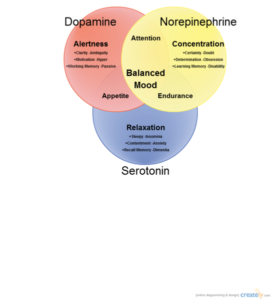 Three of these neurotransmitters- serotonin, norepinephrine, and dopamine, are integrally related to mood. Traditionally, imbalances in these chemicals have been thought to cause mood disorders such as depression and bipolar disorder. More recent studies show that acetylcholine and GABA also play a role in mood disorders.
Three of these neurotransmitters- serotonin, norepinephrine, and dopamine, are integrally related to mood. Traditionally, imbalances in these chemicals have been thought to cause mood disorders such as depression and bipolar disorder. More recent studies show that acetylcholine and GABA also play a role in mood disorders.
The ability of a pharmacologic agent to affect these neurotransmitters can signal its potential usefulness in treating mood, stress, and cognitive disorders. We’ll keep this in mind when we talk about the scientific evidence available on bacopa monnieri (BM).
Is There any Research?
As of July 2018, there are 430 articles in PubMed using the search term “Bacopa” or “Bacopa monnieri.” Only 18 of them involve human subjects. There are five clinical trials listed on ClinicalTrials.gov.
In comparison, a search on PubMed for Memantine (“Nemenda”), used for patients with dementia, brought up 3324 studies, with 301 with human subjects. There are 168 clinical trials on ClinicalTrials.gov.
Does It Improve Memory?
Bacopa monnieri (BM) has been a very important plant in Ayurvedic medicine since the time of Rig-Vega (c. 3500 BCE). It was used as a “memory tonic,” with purported anti-amnesic, memory enhancing, sedative, anti-epileptic, and anxiety relieving effects. In 1982, Singh et al[2] did some of the first studies on the effect of BM on the learning behaviors of rats. Treated or control rats underwent one of three testing protocols: (1) a shock-motivated brightness-discrimination reaction, (2) active conditioned flight reaction, or (3) continuous avoidance response.
In all three cases, rats who received BM performed significantly better than controls. Subsequently, numerous studies have been done on laboratory animals which further supports this concept and delineates some of the active nootropic components of BM (primarily the bacosides) and the neurotransmitter pathways affected by bacopa.
A 2014 article by Rajan, Preethi, and Singh [1] reviews the in vitro and animal studies that examine the neuropharmacological activity of bacopa extract. They point out studies in which BM altered acetylcholine [3, 4], serotonin [3, 5], catecholamine [6], and GABA [7, 8]. Another good review, by Aguilar and Borowski [9], summarizes the neuropharmacological effect of Bacopa in animal and in vitro studies.
Although the numbers are relatively small, there are randomized, double-blind, placebo-controlled (RDBPC) trials in humans. In 2001, Stough et al [10] did a RDBPC study with 46 healthy volunteers (ages 18-60) who received 300 mg/day of BM for 12 weeks. BM significantly improved the speed of visual information processing measured by IT (Inspection Time) test and learning rate and memory consolidation measured by the AVLT (Rey Auditory Verbal Learning Test).
The Rey Auditory Verbal Learning Test (RAVLT) evaluates a wide diversity of functions: short-term auditory-verbal memory, rate of learning, learning strategies, retroactive, and proactive interference, presence of confabulation of confusion in memory processes, retention of information, and differences between learning and retrieval.
Roodenrys et al [11] recruited 76 adults (ages 45-65) for a RDBPC trial of bacopa given for 12 weeks. Three testing sessions were held, one prior to the trial, one at the end of the trial, and one 6 weeks after completion of the trial. Each testing session consisted of a battery of tasks to assess attention, a range of memory abilities, and psychological state.
According to researchers: “The results show no significant effect of chronic administration of brahmi on measures of short-term memory, working memory, attention, or the retrieval of information from long-term memory acquired pre-experimentally.
Further, there were no significant effects on subjective measures of psychological state (depression, anxiety, and stress) or everyday memory function. There was a significant effect on a task requiring the retention of new information: the recall of unrelated word pairs after a short delay…. the effect appears to be a reduction in the amount of information lost from memory.” [11]
Calabrese et al [12] looked at the effect of BM on 54 participants (age >65) without any clinical signs of dementia. They received BM or placebo for 12 weeks. Subjects were tested using the Rey AVLT, Stroop Task*, and Divided Attention Task (DAT). ** Bacopa subjects had “enhanced” AVKT delayed word recall memory scores relative to placebo. Stroop results were also improved compared to placebo which remained unchanged. No effects were seen in the DAT.
*Stroop Task: The Stroop Effect, named after John Ridley Stroop, is a demonstration of the reaction time of a task and is often used to illustrate the nature of automatic processing versus conscious visual control. An example is how fast you can correctly say a color when the name of that color is printed in a color which is not denoted by the name: Green Red Blue Purple Red Purple.
**Divided Attention Task: Alternating attention is the ability to switch your focus back and forth between tasks that require different cognitive demands. Divided attention is the ability to process two or more responses or react to two or more different demands simultaneously. Field sobriety tests are examples of DAT.
Morgan and Stevens [13] studied 81 healthy participants over 55 years old in a RDBPC trial. All received 300 mg BM for 12 weeks. Audioverbal and visual memory performance were measured by the Rey Auditory Verbal Learning Test (AVLT), the Rey-Osterrieth Complex Figure Test (CFT)*, and the Reitan Trail Making Test (TMT)**. Bacopa significantly improved verbal learning, memory acquisition, and delayed recall as measured by the AVLT. CFT and TMT scores improved but group differences were not significant.
*Rey–Osterrieth complex figure test (ROCF): a neuropsychological assessment in which examinees are asked to reproduce a complicated line drawing, first by copying it freehand (recognition), and then drawing from memory (recall).
**Trail Making Test is a neuropsychological test of visual attention and task switching. It consists of two parts in which the subject is instructed to connect a set of 25 dots as quickly as possible while still maintaining accuracy. It's not unlike a timed version of a child's connect-the-dots puzzle.
Pase et al[14] did a systematic review of randomized controlled trials on healthy, adult humans. Six studies met their inclusion criteria (some of these studies were the ones mentioned above). The trials were 12 weeks long and used one of three Bacopa extracts at dosages of 300-450 mg/day. All reviewed trials examined the effects of Bacopa on memory, while other cognitive domains were less well studied.
The review showed that Bacopa improved performance on 9 of 17 tests in the domain of memory free recall. There was little evidence of enhancement in any other cognitive domains.
One study, by Downey et al [15], is a DBPC study on the acute effects of a special extract of Bacopa monnieri (CDRI089) on normal healthy subjects. Twenty-four subjects, ages 18-56, received 4 capsules with either placebo or 320 or 640 mg of KeenMind®(CDRI 08).
They each attended 4 sessions (one practice visit and 3 study visits) one week apart. Upon arrival, they took one cycle of CDB- a Cognitive Demand Battery- made up of a “stress and mental fatigue” visual analog scale (VAS), two Serial subtraction tasks, and the Bakan Rapid Visual Information Processing Task (RVIP).
The researchers found that “change from baseline scores indicated that the 320 mg dose of BM improved performance at the first, second and fourth repetitions of the CDB, but has no effect upon cardiovascular activity or task-induced ratings of stress and fatigue.”
Sathyanarayanan et al [16] looked at 72 healthy adults (age 35-60) who were treated with either placebo or 450 mg of BM daily for 12 weeks. Participants were tested for verbal learning, memory, attention as well as state and trait anxiety. They found no significant differences between the two groups on any of the cognitive measures although there was a “trend for lower state anxiety” in the BM group compared to placebo.
Bottom line
There is a substantial amount of in vitro and animal model research on the nootropic effects of Bacopa monnieri. However, in terms of clinical trials, the numbers are small, and the results, although significant, are not dramatic. Only certain types of memory processes may be affected. If you decide to try Bacopa to improve your memory, remember that the best effects are seen with “chronic” use, i.e. at least 12 weeks.
Does Bacopa Protect Against Acute and Chronic Stress?

According to the American Psychological Association, stress is any uncomfortable "emotional experience accompanied by predictable biochemical, physiological and behavioral changes." At times, stress can be beneficial, producing a boost that provides the drive and energy to help people get through a difficult situation, such as an exam or a work deadline.
However, an extreme amount of stress, especially over a long period of time, can have health consequences which adversely affect the immune, cardiovascular, neuroendocrine, and central nervous systems. Untreated stress can contribute to the development of psychological conditions such as depression and anxiety.
There are several articles which look at the effect of bacopa on anxiety, mood, and/or depression. Chatterjee et al [17]looked at the effect of Bacopa monnieri and Panax quinquefolium (Ginseng) in experimental anxiety and depression models in mice. Mice were divided into treatment or placebo groups and were treated with either bacopa or ginseng at three different doses (8 mice in each), or Imipramine or diazepam- standard treatment for depression/anxiety, respectively.
Anxiety was studied through a battery of tests: light/dark test, elevated plus maze test and holeboard test. Depression experiments were conducted following tail suspension test and forced swim test. They also performed a rotarod test to study motor incoordination. In the anxiety models, mice receiving 80mg/kg of BM showed significant improvement in the “elevated plus maze” and “light/dark” tests compared to controls, but not to the same degree as those treated with diazepam.
(BTW- the labels of Fig. 3 and 4 in this paper are reversed, leading to a fair amount of temporary confusion on my part). In the depression models, BM showed significant improvement in both tests, at varying doses compared to control mice. Again, not to the same degree as when mice are treated with a standard antidepressant. It is interesting to note that Ginseng did better on all tests than BM.
Rai et al[18] looked at bacopa as an adaptogen- an anti-stress agent, in rats. They used an immobilization test to stress the rats, either on a one-time (acute) basis (AS) or for a week- chronic stress (CS). Rats were treated with BM or ginseng for 3 days for AS or 45 minutes before each exposure in CS.
There were also two control groups who received either no stress or AS or CS without treatment. All rats were sacrificed afterwards and were tested for (1) weight of adrenal gland, spleen and thymus, (2) ulcer index, and (3) blood chemistries including glucose, liver function, cholesterol panel, and CK (creatinine kinase). Their results are as follows:
“AS exposure significantly increased the ulcer index, adrenal gland weight, plasma glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatine kinase (CK) but significantly decreased the spleen weight. Pretreatment with B. monniera at 40 mg/kg po significantly reduced the AS-induced increase in the ulcer index, adrenal gland weight, plasma glucose, AST, and CK. A dose of 80 mg/kg po significantly reversed the AS-induced changes in adrenal gland weight, spleen weight, plasma glucose, ALT, and AST. Pretreatment with low dose of B. monniera extract at 40 mg/kg significantly reversed changes in ulcer index and plasma AST only, whereas the pretreatment with higher dose significantly reversed CS-induced changes in ulcer index, adrenal gland weight, CK, and AST.” [18]
One of the more interesting animal models was in a paper by Phulara et al [19], who studies the effects of BM on the nematode C. elegans under stress conditions. Who knew that a worm could get stressed out? Bacopa significantly enhanced stress tolerance and increased the mean lifespan of worms during thermal and oxidative stress.
There are three studies that look at the effect of bacopa on stress in humans. Stough et al [10] (see above), also performed a state anxiety test (State-Trait Anxiety Inventory) on his subjects. They found that BM produced a significant decrease in state anxiety. State anxiety is defined as unpleasant feelings when confronted with specific situations, demands or a particular object or event. When the object or situation that is perceived as threatening goes away, the person no longer experiences anxiety.
Likewise, Calabrese [12] also tested his subjects with the State-Trait Anxiety Inventory (STAI) as well as the Center of Epidemiologic Studies Depression scale (CESD-10)and the Profile of Mood States test. There was no significant improvement in depressive scales in patients treated with bacopa. There was some improvement of anxiety scores with bacopa vs. placebo, but the researchers combined both trait and state measurements into a single score, and the effect they found remained unchanged between their first and second follow-up visits.
Downey et al [15, 20] did a DBPC cross-over study using the bacopa extract KeenMind®(CDRI 08) in 17 healthy volunteers. In addition to cognitive testing, the researchers used the State Trait Anxiety Inventory and Bond-Lader VAS test to evaluate mood and measured salivary cortisol levels at baseline and 1 and 2 hours after consuming a placebo, 320 mg BM or 640 mg BM.
Cortisol levels declined slightly in a dose-dependent fashion (640mg > 320mg) compared to placebo. However, “subjective ratings of mood and anxiety were not consistently affected by BM consumption (although Alertness and Contentedness ratings were increased).”
Bottom line
As in the case of memory enhancement, there is some evidence that Bacopa may have adaptogenic effects in acute and chronic stress conditions. But the results are not dramatic and there is a definite need for larger trials in human subjects before bacopa can be recommended as a treatment for stress.
Does it Help Alzheimer’s Disease or Dementia?
Dementia is the loss of cognitive functioning—thinking, remembering, and reasoning—and behavioral abilities to such an extent that it interferes with a person's daily life and activities. These functions include memory, language skills, visual perception, problem-solving, self-management, and the ability to focus and pay attention. Alzheimer's disease (AD) is the most common cause of dementia in older adults. Other dementias include Lewy body dementia, frontotemporal disorders, and vascular dementia
The exact cause of AD is uncertain but there is a general consensus about some of the factors that may be involved. Oxidative stress plays a major role in the severity of the disease. Oxidative stress is an imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects through neutralization by antioxidants.
These can be endogenous antioxidants (made by the body) such as reduced glutathione (GSH), superoxide dismutase (SOD) and nitric oxide (NO), or exogenous (supplemented by diet or other means). Deficits in acetylcholine are also seen in dementia patients. Accumulating evidence suggests an important role of chronic inflammation in the development of dementia, with the involvement of immune system components such as cyclooxygenase (COX) 1 and 2, and prostaglandins.
Viji et al [21] found that BM can inhibit LOX (lipoxygenase), COX-2 and decrease TNF (tumor necrosis factor) in rat monocytes in vivo and human whole blood in vitro. Russo et al [22] showed that BM can reduce reactive oxygen species and inhibit nitrous oxide-induced cellular alterations in cultured rat astrocytes.
In animal models, Saini, Singh, and Sandhir [23] measured the effect of BM on the cognitive impairment induced in rats by the administration of colchicine. Cognitive impairment was accompanied by a significant increase in oxidative stress as measured by lipid peroxidation and protein carbonyls. BM supplementation reversed the memory impairment in colchicine treated rats along with the restoration of lipoperoxidase and protein carbonyl levels.
Khan et al [24] showed that streptozotocin deteriorated memory and learning skills in rats. They also visualized thiobarbituric acid reactive substances, indicative of lipid peroxidation, in the brains of these rats. Finally, they found a disturbance of antioxidant status in the hippocampus, along with decreases in copper and zinc in these animals.
All these abnormalities were significantly improved by Brahmi supplementation. They conclude that "the data suggests that formation of free radicals and increased rate of lipid peroxidation as a result of streptozotocin induction might cause neurotoxicity in these animals which can be prevented by the use of Brahmi." [25]
There are currently no human studies that investigate the effect of BM on patients with Alzheimer’s disease or dementia. As mentioned above, there are some double-blind placebo-controlled studies looking at the effect of BM on memory [13, 26], however, these studies are all done on patients without any signs of AD or dementia.
Bottom line
Animal studies and in vitro testing shows that bacopa has antioxidant effects and can reverse dementia-like changes induced in animal models. However, there are, as of yet, no human studies on patients with dementia or Alzheimer’s Disease. Large scale DBPC studies are needed.
Can it Treat ADHD?
Attention-deficit/hyperactivity disorder (ADHD) is a brain disorder marked by an ongoing pattern of inattention and/or hyperactivity-impulsivity that interferes with functioning or development. A meta-analysis of 175 research studies worldwide on ADHD prevalence in children aged 18 and under found an overall pooled estimate of 7.2% (Thomas et al. 2015).
The US Census Bureau estimates 1,795,734,009 people were aged 5-19 worldwide in 2013. Thus, 7.2% of this total population is 129 million—a rough estimate of the number of children worldwide who have ADHD.[27]
There are four papers which discuss the use of bacopa extract in the treatment of children and/or adolescents with ADHD. A 2014 open-label study by Dave et al [28] included 31 children between 6 and 12 years of age diagnosed with ADHD. All children received a dose of 225mg/day of BM for 6 months. They were evaluated using a Patent Rating Scale to assess ADHD symptoms at baseline and at the end of the treatment period. Their results (only available as an abstract) were as follows:
“SBME significantly reduced the subtests scores of ADHD symptoms, except for social problems. The symptom scores for restlessness were reduced in 93% of children, whereas improvement in self-control was observed in 89% of the children. The attention-deficit symptoms were reduced in 85% of children.
Similarly, symptom scores for learning problems, impulsivity, and psychiatric problems were reduced for 78%, 67%, and 52% of children, respectively. It was observed that 74% of the children exhibited up to a 20% reduction, while 26% of children showed between a 21% and a 50% reduction in the total subtests scores.” [3]
A 2016 paper by Kean et al [29] does a systematic meta-analysis of Bacopa in child and adolescent populations. Out of the 5 studies which met the inclusion criteria, only two make any mention of studying the effect of the herb on the symptoms of ADHD. One of the papers is the Dave et al [3] article above, and the second by Lucknow et al[30].
This study, a double-blind, randomized trial, was conducted at the Department of Pediatrics, BRD Medical College, Gorakhpur, India. Nineteen children, aged 8-10 years old, diagnosed with ADHD were given 50 mg. of Bacopa twice daily. 17 ADHD children received a placebo. After 12 weeks of treatment, the children took a battery of specialized tests.
The data revealed a significant improvement in the areas of sentence repetition, logical memory, and pair-associative learning (matching things that go together; e.g., “test” and “grade”) in all 19 children who took Bacopa.
The evaluation did not occur until four weeks after stopping Bacopa usage, indicating that it had a lasting effect. According to Dr. O. P. Asthana, head of the pediatric department, there were no side effects and the herb was very well tolerated. This original paper is not available for review, so I don’t know what its findings relating to ADHD symptoms showed.
The last paper, another by Kean et al [31] in 2017 evaluates a multiherbal formula (24 herbs in all) called Mentat. Bacopa extract is the predominant herb in the formula. They evaluated 6 studies which used Mentat to treat children and adolescents with a variety of behavioral or mental conditions.
Two studies involved subjects with ADHD. Although the results may show some improvement in cognitive functions, the results of both studies regarding ADHD symptoms are listed as N/A: i.e. “not available.”
Bottom Line
There is not enough evidence to support the use of bacopa for the treatment of ADHD.
Can it Reduce Symptoms of Epilepsy or Schizophrenia?
Epilepsy is a brain disorder that causes people to have recurring seizures. The seizures happen when clusters of nerve cells, or neurons, in the brain send out the wrong signals. People may have strange sensations and emotions or behave strangely. They may have violent muscle spasms or lose consciousness.
Epilepsy can have many possible causes. Anything that disturbs the normal pattern of neuron activity—from illness to brain damage to abnormal brain development—can lead to seizures. Epilepsy may develop because of an abnormality in brain wiring, an imbalance of nerve signaling chemicals called neurotransmitters, changes in important features of brain cells called channels, or some combination of these and other factors.
GABA (gamma amino butyric acid) is the main inhibitory neurotransmitter in the cerebral cortex. It maintains an inhibitory effect which counter balances nerve excitation Seizures can occur if this balance is upset.
A study by Matthew et al [32] looked at GABA receptors in the brains of epileptic rats and whether bacopa can have a therapeutic effect on those receptors. Rats were divided into 5 categories: control (non-epileptic), epileptic, epileptic treated with bacopa, epileptic treated with bacoside A, epileptic treated with carbamazepine (a drug used to treat seizures).
All treated rats received medications for 15 days. Rats were tested for cognitive/ memory effects using a radial arm test. A Y-maze test quantified depression-like and anxiety-like behavior. All the rats were sacrificed at the end of the study and their brains were imaged and assayed for GAD (glutamate decarboxylase- an enzyme that converts glutamate to GABA), GABA receptors, and GABA receptor subunit mRNA.
Rats treated with bacopa or bacoside A showed results in nearly all tests like those of controls or those treated with carbamazepine. Their findings support the concept that decreased GABA receptors and GAD activity in the cerebral cortex play an important role in seizure initiation and mood disorders associated with epilepsy.
Some patients with epilepsy have been shown to have cognitive and behavioral impairments and other comorbid conditions, such as depression and anxiety are increasingly recognized within a broad spectrum of health impacts associated with epilepsy. These comorbid conditions may be caused by similarities in hereditary, structural, chemical or neurotransmitter abnormalities between the conditions.
Drugs used to treat seizures can also cause cognitive and behavioral impairment. A study by Vohora, Pal, and Pillai [33] looked at the effect of bacopa on cognitive impairment associated with the treatment of epilepsy with phenytoin. BM was evaluated both alone and in combination with phenytoin in mice. They looked at three different activities- (a) passive-avoidance (PA) task; (b) maximal electroshock seizures; and (c) locomotor activity.
Phenytoin (PHT) adversely affected cognitive function in the PA task. BM extract was given along with phenytoin in the second week of the two-week regimen, significantly reversed PHT-induced impairment. Both acquisition and retention of memory showed improvement without affecting its anticonvulsant activity.
At present, there are no studies in humans which look at the ability of BM to reduce the primary or comorbid symptoms of epilepsy.
Schizophrenia is a chronic and severe mental disorder that affects how a person thinks, feels, and behaves. People with schizophrenia may seem like they have lost touch with reality. Although schizophrenia is not as common as other mental disorders, the symptoms can be very disabling.
They include “positive” symptoms such as hallucinations, delusions, thought and movement disorders, “negative” symptoms such as “flat affect” lack of pleasure in everyday life, lack of ability to begin and sustain planned activities and cognitive symptoms- poor “executive functioning” (the ability to understand information and use it to make decisions), trouble focusing or paying attention and problems with “working memory” (the ability to use information immediately after learning it).
Two groups of researchers have looked at the effect of bacopa in a rat model of schizophrenia. Chatterjee et al [17] looked at the effect of BM, in graded doses (40mg, 80mg, and 120mg/kg) on mice given ketamine- drug which is known to induce psychosis in mice. The mice were given tests designed to measure a few parameters involving both positive, negative, and cognitive symptoms.
They also had a subgroup of mice who received BM on a “chronic” basis of 10 days and tested for negative symptoms. Lastly, they looked at the levels of serotonin, glutamate, acetyl-cholinesterase and monoamine oxidase in the brains of the mice. Based on their results, they concluded that BM had a protective effect against ketamine-induced positive and negative symptoms as well as the cognitive dysfunctions.
Furthermore, the antipsychotic properties may be related to its normalization of dopamine and serotonergic neurotransmission and reduction of acetylcholinesterase activity.
Two papers by Piyabhan et al [34, 35] investigated the effects of bacopa in a rat model of schizophrenia induced with phencyclidine (PCP). Rats treated with BM were able to recover cognitive ability lost by PCP. Additional testing showed that the improvement was mediated by changes in the regulation of glutamate.
The only case of BM treatment of human subjects with schizophrenia was a case report by Sarkar et al [36]. A 34-yr-old man with schizophrenia of 15 years duration was treated with olanzapine (a drug used to treat the symptoms of schizophrenia). Prior to the treatment, he had a positive and negative syndrome scale (PANSS) of 108.
After two weeks on olanzapine, his PANSS score was 92, but his improvement of symptoms decreased by week 3. After 5 weeks of treatment, 250mg of Brahmi was added twice a day to his regimen. One month later, his PANSS score had dropped to 67.
Bottom line
Although there are some promising results in mice and rats, there are no placebo controlled, double blind studies in humans to support the use of BM in the treatment of epilepsy or schizophrenia.
Does it Reduce Blood Pressure?
I could find no studies which looked at the effect of BM on blood pressure. I found three which looked at BM’s effect on blood pressure in rats. In two [37, 38], there was no significant effect on blood pressure, even when given on a chronic basis [3]. An earlier paper by Kamkaew et al [39] showed a dose-related decrease in systolic and diastolic blood pressure in anesthetized rats. The drop was short-lived and returned to normal within one minute.
Bottom Line
There is no evidence to support the use of bacopa to treat high blood pressure.
Is Bacopa Safe?
Phase I studies of a drug are usually the first that involve people. The main reason for doing phase I studies is to find the highest dose of the treatment that can be given safely without serious side effects. They are not designed to find out if the treatment works. A Phase 1 study on bacopa was done by Pravina et al [40]. They looked at BacoMind ™, an “enriched phytochemical composition developed from B. monnieri."
Twenty-three volunteers were given one capsule of BacoMind™ daily for 30 days, 300 mg for the first 15 days, and 450 mg for the next 15. Clinical, hematological, biochemical and electrocardiographic parameters were measured before and after treatment. They reported no untoward effects in the volunteers.
Mild adverse effects were related to the gastrointestinal system-nausea, increased intestinal motility, and gastrointestinal upset. The symptoms resolved spontaneously, without the need to discontinue the treatment.
Bottom line
Bacopa has been used for many years in traditional medicine and appears to be safe for human consumption. The most common side effects are related to the GI tract.
Conclusion
Bacopa monnieri has been used as a “mental tonic” for hundreds of years in Ayurvedic and Traditional Chinese medicine. Over the past thirty years, a number of researchers have isolated the active ingredients of BM and have begun to characterize how these compounds interact with neurotransmitters, receptors and even mRNA.
The hope is that bacopa can play a role in the treatment of cognitive and emotional disturbances. Unfortunately, there are few clinical trials in humans and the results have not been dramatic. Larger RDBPC studies are necessary before a recommendation for the use of this drug as a treatment option can be made.
Overall, bacopa is a relatively safe supplement. However, remember that if you wish to try it as a memory enhancer, you may not see any improvements for several months.

Leave a Reply